By DANA TIMS/YachatsNews.com
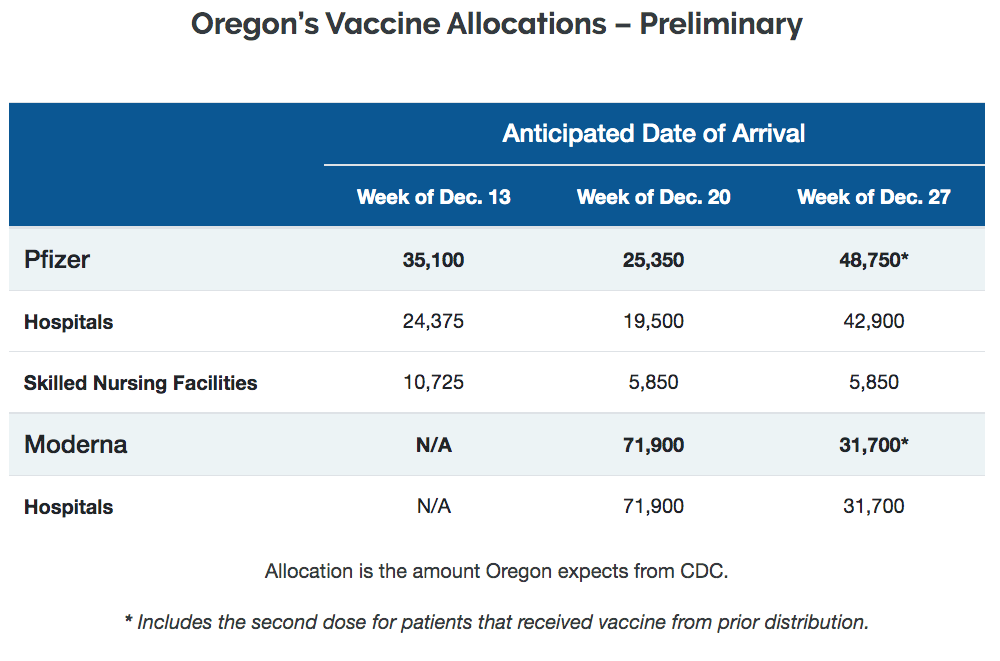
The first doses of a groundbreaking vaccine deemed effective at combating COVID-19 arrived Thursday in Lincoln County.
The arrival means that within a day or two, a hastily assembled network of medical professionals and providers will swing into action in an effort to halt a virus that has infected tens of thousands in Oregon and millions more across the world.
“This is a very exciting time for us at Samaritan Health Services and the community as a whole,” Dr. Adam Brady, a Samaritan infectious disease specialist, said during a televised seminar Thursday afternoon. “It’s been a very rough year and a difficult time for everyone, but what this shows is that there’s light at the end of the tunnel.”
There remain many unknown factors about exactly how, where and when Lincoln County’s vaccine rollout will occur, Brady said. He urged members of the public to stay tuned to announcements from various agencies, including Lincoln County’s health department and Samaritan Health Services to get information as it becomes available.
The initial vaccination schedule includes three tiers of recipients, all selected on a prioritized basis of need and risk.
The first group, labeled 1A recipients, includes health care workers, with first distribution to those with direct exposed to COVID-19 in their work. These include, for instance, hospital paid and unpaid workers, from clinicians to maintenance specialists, EMS, and long-term care facilities employees and residents. Brady said the goal is to complete this first phase by the end of January.
The second tier, labeled 1B, includes teachers, bus drivers, food processors and other people deemed “essential” to keeping both society and the economy moving.
The third tier, 1C, includes people with underlying health conditions and people over the age of 65. The goal for vaccinating the latter two groups is late spring, depending on vaccine availability.
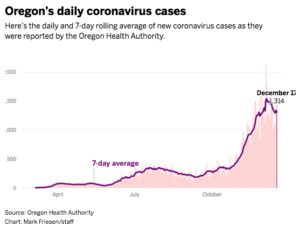
As of Thursday, COVID-19 was racking up record numbers of both new infections and deaths in the state. A total of 48 people died Wednesday alone, according to Oregon Health Authority figures, bringing the state’s death total to 1,262. Total infections across Oregon neared 100,000, meaning that even an aggressive vaccination campaign will take months to slow what has been an ever increasing rate of new infections and deaths.
Still, Brady hailed the arrival of the first vaccine — manufactured by pharmaceutical giant Pfizer and BioNTech, a German firm – noting that it’s the first vaccine approved by the federal government, albeit on an emergency-use basis, to fight COVID-19.
“The best thing people can do is get vaccinated when it’s available,” he said, “so we can get life back to normal.”
Planning for vaccination clinics
Samaritan’s five hospitals, located in Lincoln, Linn and Benton counties, will work with county health departments and other medical providers to implement a vaccination strategy outlined by both the Oregon Health Authority and the federal Centers for Disease Control, Brady said.
“Plans are now being made for mass vaccination clinics,” he said, adding that retired medical professionals should “stay tuned” for possible calls for volunteers.
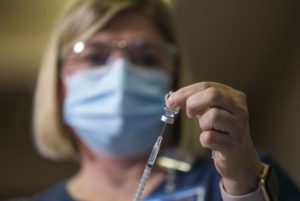
A few hundred doses of the vaccine have already been distributed to hospitals in Portland, Tualatin and Ontario. The remaining 30,255 Pfizer vaccine doses from this week’s initial allotment of 35,100 doses for Oregon will arrive at hospitals throughout the rest of the week, according to state health officials.
An additional 10,725 doses will go to skilled nursing facilities for vaccinations scheduled to start next week.
A shortage of the vaccine means it could take months before everyone who wants to get a shot will actually have one available, Brady said. In the meantime, he urged people to continue wearing masks and practicing social distancing as strategies to curb the disease’s spread.
Brady spent most of the hour-long session explaining how the new virus works, and taking questions from people watching the session. What follows are questions and answers not only from Brady, but from other medical professionals who have spent time examining the effort to produce vaccines intended to prevent what, so far, has been the devastating spread of COVID-19 around the world.
Question: What do I need to know about the COVID-19 vaccine that’s just been approved by the U.S. government?
Answer: The vaccine was developed, in record time, by pharmaceutical giant Pfizer and BioNTech, a German firm. To be fully effective, it requires two shots, given three weeks apart. It’s also the first vaccine using messenger RNA technology ever approved for human use by the federal Food and Drug Administration. Traditional vaccines rely on a dead or weakened virus to essentially tell the body’s immune system how to recognize infected cells. The new vaccine uses a lab-manufactured piece of genetic information that instructs your cells to create a protein, which in turns activates an immune response to kill the pathogen if it tries to invade.
Q: How do we know it’s both safe and effective?
A: 44,000 people participated in randomized clinical trials, in which one group received two doses of the vaccine and the other group received placebos. Follow-up studies showed that the vaccine proved 95 percent effective in preventing participants from getting COVID-19. Only eight of the more than 20,000 people who got both doses contracted the virus. Among the placebo group, by comparison, 162 people contracted the virus.
Q: Are there side effects?
A: Oregon health authorities, who reviewed the dozens of pages of data from Pfizer’s clinical trials, said it’s common for those vaccinated to have a sore arm or red spot at the vaccination point, feel fatigued, have chills and a low-grade fever. But fewer than 5 percent wouldn’t be able to go to work the next day. For this reason, front-line health care workers receiving the virus first will be vaccinated on a staggered basis to avoid large-scale numbers of people not being able to report for work in hospitals and clinics.
Q: Are other vaccines, manufactured by other companies, likely to get approved?
A: Yes. Moderna, a Massachusetts-based vaccine developer, and AstraZeneca, a huge British biopharmaceutical company, are in the late stages of making a COVID-19 vaccine. Federal government approval could come at any time, especially for Moderna’s new vaccine.
Q: What are the key differences among the three?
A: Pfizer’s vaccine is the most problematic because it must be stored at -94 degrees. That means ultra-cold storage units are needed to keep it viable. And once it’s unthawed, the vaccine has a shelf life of only one week. Moderna’s vaccine can be kept in a regular freezer and has a shelf life of one month after it’s thawed. AstraZeneca’s version can be stored at temperatures between 36 and 46 degrees and has a shelf life of six months.
Q: What process is Oregon using to address vaccine allocations?
A: Front-line health care workers, along with residents and workers in long-term care facilities make up what the Oregon Health Authority refers to as the 1a group. An estimated 400,000 Oregonians fall into this category. Guidelines containing more specifics about how this group will be vaccinated are being prepared. Officials say it could take a few months to vaccinate everyone in the 1a “essential worker” category. After that, a vaccine-distribution advisory committee will decide who falls into groups that will be vaccinated next.
Q: How soon will “every day people” be able to get vaccinated?
A: Health officials say that the expected ongoing shortage of vaccines makes it difficult to predict when the general population will have access. Susan Trachsel, public information officer for Lincoln County Public Health said May or June might be reasonable targets, but she added that the entire process is so new that predictions are hard to make at this point.
Q: Will all parts of the state receive vaccine shipments?
A: Yes. Health authority officials intend to distribute the vaccine to hospitals in each county based on its population.
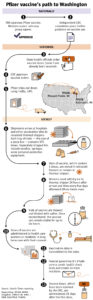
Q: Since the Pfizer vaccine is already being administered, how will its storage requirements effect its distribution?
A: State health officials note that most of the ultra-cold storage units needed for the vaccine are located in populated areas near Portland and along the Interstate 5 corridor. To ensure equitable distribution, they are recruiting hospitals in rural areas to serve as hubs. They are also working to acquire an additional 20 or so ultra-cold storage units to place in less populated areas around the state.
Q: From what we know, are Oregonians willing to be vaccinated?
A: A recent survey showed that almost half of Oregon residents weren’t certain they would get vaccinated. That’s far below the 70 percent level that state health officials say is needed to achieve so-called ‘herd immunity’ – a concept in which a population can be protected from a certain virus if a threshold of vaccination is reached.
Q: Are there strategies being developed to boost that 50 percent figure?
A: Yes. State and local officials are working to get stories about that stress the vaccines’ safety. The governor’s office is also directing a major paid media campaign stressing the benefits of vaccination. In addition, health officials are recruiting so-called social media influencers to help spread a pro-vaccination message among communities that historically have been resistant to large-scale use of vaccines.